Subject: FLUORIDE BATTLE RAGING IN AIKEN,
SC...11-22-12
FORWARDED for Education, Edification, and Information Purposes Only
Not spam - contact: becworks@gmail.com
++++++++++++++++++++
Scott:
[Responding]
Below is a sixth grade level of information on fluoride, with references to the industrial chemistry you lack...grow up...the nation poisoned entire generations of children...to gain control over the future...that was what Goals 2000 was all about. I know, because I studied it at the University of South Carolina in my graduate studies in the 1990s. You lack science knowledge. The universities are hell-bent on protecting the agenda, as is evident in the tweaked info on safety in public documents...it is safe but toxic...we are playing Russian Roulette with children's lives...and you are an accomplice to that agenda.
READ the following and learn. Then, read the MSDS for sodium fluoride. Then, read chemistry on fluoride, the most aggressive element in the periodic chart, because it replaces all elements beneath it on the chart. Fluoride is not meant for human bodies.
Sincerely,
R.E. Sutherland
QUOTE:
FORWARDED for Education, Edification, and Information Purposes Only
Not spam - contact: becworks@gmail.com
++++++++++++++++++++
Scott:
[Responding]
Below is a sixth grade level of information on fluoride, with references to the industrial chemistry you lack...grow up...the nation poisoned entire generations of children...to gain control over the future...that was what Goals 2000 was all about. I know, because I studied it at the University of South Carolina in my graduate studies in the 1990s. You lack science knowledge. The universities are hell-bent on protecting the agenda, as is evident in the tweaked info on safety in public documents...it is safe but toxic...we are playing Russian Roulette with children's lives...and you are an accomplice to that agenda.
READ the following and learn. Then, read the MSDS for sodium fluoride. Then, read chemistry on fluoride, the most aggressive element in the periodic chart, because it replaces all elements beneath it on the chart. Fluoride is not meant for human bodies.
Sincerely,
R.E. Sutherland
QUOTE:
Sodium fluoride
From Wikipedia, the free
encyclopedia
Jump to: navigation, search
|
Sodium
fluoride
|
|
|
IUPAC
name[hide]
Sodium fluoride
|
|
|
Other
names[hide]
Florocid
|
|
|
Identifiers
|
|
|
1690
|
|
|
WB0350000
|
|
|
[show]
|
|
|
Properties
|
|
|
NaF
|
|
|
41.988173 g/mol
|
|
|
Appearance
|
White solid
|
|
odorless
|
|
|
2.558 g/cm3
|
|
|
993 °C, 1266 K, 1819 °F |
|
|
1704 °C, 1977 K, 3099 °F |
|
|
Solubility
in water
|
36.4 (0 °C); 40.4 (20 °C); 50.5 (100 °C) g/L[1]
|
|
1 mmHg @ 1077 C°[2]
|
|
|
Refractive
index (nD)
|
1.336
|
|
Structure
|
|
|
cubic
|
|
|
Hazards
|
|
|
EU Index
|
009-004-00-7
|
|
0
3
0
|
|
|
Non-flammable
|
|
|
52–200 mg/kg (oral in rats, mice, rabbits)[3]
|
|
|
Related
compounds
|
|
|
Other anions
|
|
|
Other cations
|
|
|
Related compounds
|
|
|
Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
Contents
|
Structure, general properties, occurrence
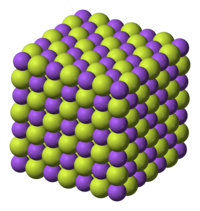
Sodium
fluoride is an ionic
compound, dissolving to give separated Na+ and F−
ions. Like sodium
chloride, it crystallizes in a cubic motif where both Na+ and F−
occupy octahedral
coordination sites;[4][5]
its lattice spacing, approximately 462 pm, is somewhat smaller than that of
sodium chloride.The mineral form of NaF, villiaumite, is moderately rare. It is known from plutonic nepheline syenite rocks.[6]
Production
NaF
is prepared by neutralizing hydrofluoric acid or
hexafluorosilicic
acid (H2SiF6), byproducts of the production of superphosphate fertilizer.
Neutralizing agents include sodium hydroxide and sodium carbonate.
Alcohols are sometimes used to precipitate the NaF:
HF + NaOH → NaF + H2O
From
solutions containing HF, sodium fluoride precipitates as the bifluoride salt NaHF2.
Heating the latter releases HF and gives NaF.
HF + NaF ⇌ NaHF2
In
a 1986 report, the annual worldwide consumption of NaF was estimated to be
several million tonnes.[7]
Applications
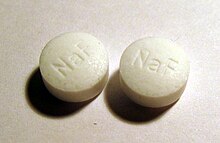
Sodium fluoride is sold
in tablets for cavity prevention.
Fluoride
salts are used to enhance the strength of teeth by the formation of fluorapatite, a naturally
occurring component of tooth enamel.[8][9]
Although sodium fluoride is also used to fluoridate water
and, indeed, is the standard by which other water-fluoridation compounds are
gauged, hexafluorosilicic
acid (H2SiF6) and its salt sodium hexafluorosilicate
(Na2SiF6) are more commonly used additives in the U.S.[10]
Toothpaste often contains
sodium fluoride to prevent cavities.[11]
Alternatively, sodium fluoride is used as a cleaning agent, e.g. as a
"laundry sour".[7]
A variety of specialty chemical applications exist in synthesis and extractive metallurgy. It reacts with
electrophilic chlorides including acyl chlorides, sulfur
chlorides, and phosphorus chloride.[12]
Like other fluorides, sodium fluoride finds use in desilylation in organic synthesis.
The fluoride is the reagent for the synthesis of fluorocarbons.In medical imaging, fluorine-18-labelled sodium fluoride is used in positron emission tomography (PET). Relative to conventional bone scintigraphy carried out with gamma cameras or SPECT systems, PET offers more sensitivity and spatial resolution. A disadvantage of PET is that fluorine-18 labelled sodium fluoride is less widely available than conventional technetium-99m-labelled radiopharmaceuticals.
Safety
See
also: Fluoride poisoning
The lethal dose for a 70 kg (154 lb) human is
estimated at 5–10 g.[7]
Sodium fluoride is classed as toxic by both
inhalation (of dusts or
aerosols) and ingestion.[13]
In high enough doses, it has been shown to affect the heart and circulatory
system.In the higher doses used to treat osteoporosis, plain sodium fluoride can cause pain in the legs and incomplete stress fractures when the doses are too high; it also irritates the stomach, sometimes so severely as to cause ulcers. Slow-release and enteric-coated versions of sodium fluoride do not have gastric side effects in any significant way, and have milder and less frequent complications in the bones.[14] In the lower doses used for water fluoridation, the only clear adverse effect is dental fluorosis, which can alter the appearance of children's teeth during tooth development; this is mostly mild and is unlikely to represent any real effect on aesthetic appearance or on public health.[15]
See also
References
1.
^ Haynes, William M., ed. (2011). CRC
Handbook of Chemistry and Physics (92nd ed.). CRC Press. p. 5.194. ISBN 1439855110.
2.
^ Lewis, R.J. Sax's Dangerous Properties of
Industrial Materials. 10th ed. Volumes 1–3 New York, NY: John Wiley & Sons
Inc., 1999., p. 3248
3.
^ Martel, B.; Cassidy, K. (2004), Chemical
Risk Analysis: A Practical Handbook, Butterworth–Heinemann, p. 363, ISBN 1-903996-65-1
4.
^ Wells, A.F. (1984), Structural Inorganic
Chemistry, Oxford: Clarendon
Press, ISBN 0-19-855370-6
5.
^ "Chemical and
physical information" (PDF), Toxicological profile for fluorides,
hydrogen fluoride, and fluorine, Agency for Toxic Substances and Disease
Registry (ATDSR), September 2003, pp. 187, retrieved 2008-11-01
7.
^ a
b
c
Aigueperse, Jean; Paul Mollard, Didier Devilliers, Marius Chemla, Robert Faron,
Renée Romano, Jean Pierre Cuer (2005), "Fluorine Compounds,
Inorganic", in Ullmann, Encyclopedia of Industrial Chemistry,
Weinheim: Wiley-VCH, doi:10.1002/14356007.a11_307
8.
^ Bourne, Geoffrey Howard (1986), Dietary research and
guidance in health and disease, Karger, p. 153, ISBN 3-8055-4341-7,
Snippet
view from page 153
9.
^ Klein, Cornelis; Hurlbut, Cornelius Searle;
Dana, James Dwight (1999), Manual of Mineralogy (21 ed.), Wiley, ISBN 0-471-31266-5
10.
^ Division of Oral Health, National Center for
Prevention Services, CDC (1993) (PDF), Fluoridation
census 1992, retrieved 2008-12-29.
11.
^ "Sodium
fluoride, Molecule of the week". American
Chemical Society. 2008-02-19. Retrieved 2008-11-01.
12.
^ Halpern, D.F. (2001), "Sodium
Fluoride", Encyclopedia of Reagents for Organic Synthesis, John Wiley &
Sons, doi:10.1002/047084289X.rs071
14.
^ Murray TM, Ste-Marie LG. Prevention and
management of osteoporosis: consensus statements from the Scientific Advisory
Board of the Osteoporosis Society of Canada. 7. Fluoride therapy for
osteoporosis. CMAJ. 1996;155(7):949–54. PMID 8837545.
15.
^ National Health and Medical Research Council
(Australia). A
systematic review of the efficacy and safety of fluoridation [PDF].
2007. ISBN
1-86496-415-4. Summary: Yeung CA. A systematic review of the efficacy and
safety of fluoridation. Evid Based Dent. 2008;9(2):39–43. doi:10.1038/sj.ebd.6400578. PMID 18584000.
Lay
summary: NHMRC, 2007.
|
|
[show]
Mineral supplements (A12)
END QUOTE
+++++++++++++++++++++++++++++++++++++++
END QUOTE
+++++++++++++++++++++++++++++++++++++++
On Thu, Nov 22, 2012 at
11:57 PM, Scott Bergeson <scottb@xmission.com>
wrote:
Quoting R.E. Sutherland
on Thu, 22 Nov 2012 19:09:05 -0500:
Hello Scott,
The element Fluoride is different from the compound Sodium Fluoride.
Apparently you are lacking in science knowledge.
The compound form of fluoride called sodium
fluoride is found in drinking water and toothpaste.
Now, go look up the MSDS for "SODIUM FLUROIDE" ... and get better educated.
Sincerely,
R.E. Sutherland, M.Ed.
-----
Thank you for your vote of confidence.
The attached PDF file came up first in a startpage search.
https://www.sciencelab.com/msds.php?msdsId=9927595
Zero occurrences. As it should be. MSDS writers have better
science education than you. And while not relevant to
whether fluoride be a "toxin"* (really weird theology you
have there, to not only call stars living organisms, but
hold they make and secrete fluorine for their biological
defense), ions are ions. Fluoride is an anion formed
when a fluorine atom (½ of a fluorine molecule) takes
an additional electron to complete its valence octet.
When dissociated, the counterion makes no difference.
* Toxin: a toxic substance - poison - an organism makes and secretes.
Now, if you expect people to take concerns about such bad actors as
fluoride seriously, it would help to lose the fringy misdefinitions.
Hello Scott,
The element Fluoride is different from the compound Sodium Fluoride.
Apparently you are lacking in science knowledge.
The compound form of fluoride called sodium
fluoride is found in drinking water and toothpaste.
Now, go look up the MSDS for "SODIUM FLUROIDE" ... and get better educated.
Sincerely,
R.E. Sutherland, M.Ed.
-----
Thank you for your vote of confidence.
The attached PDF file came up first in a startpage search.
https://www.sciencelab.com/msds.php?msdsId=9927595
Zero occurrences. As it should be. MSDS writers have better
science education than you. And while not relevant to
whether fluoride be a "toxin"* (really weird theology you
have there, to not only call stars living organisms, but
hold they make and secrete fluorine for their biological
defense), ions are ions. Fluoride is an anion formed
when a fluorine atom (½ of a fluorine molecule) takes
an additional electron to complete its valence octet.
When dissociated, the counterion makes no difference.
* Toxin: a toxic substance - poison - an organism makes and secretes.
Now, if you expect people to take concerns about such bad actors as
fluoride seriously, it would help to lose the fringy misdefinitions.



No comments:
Post a Comment